PIPELINE
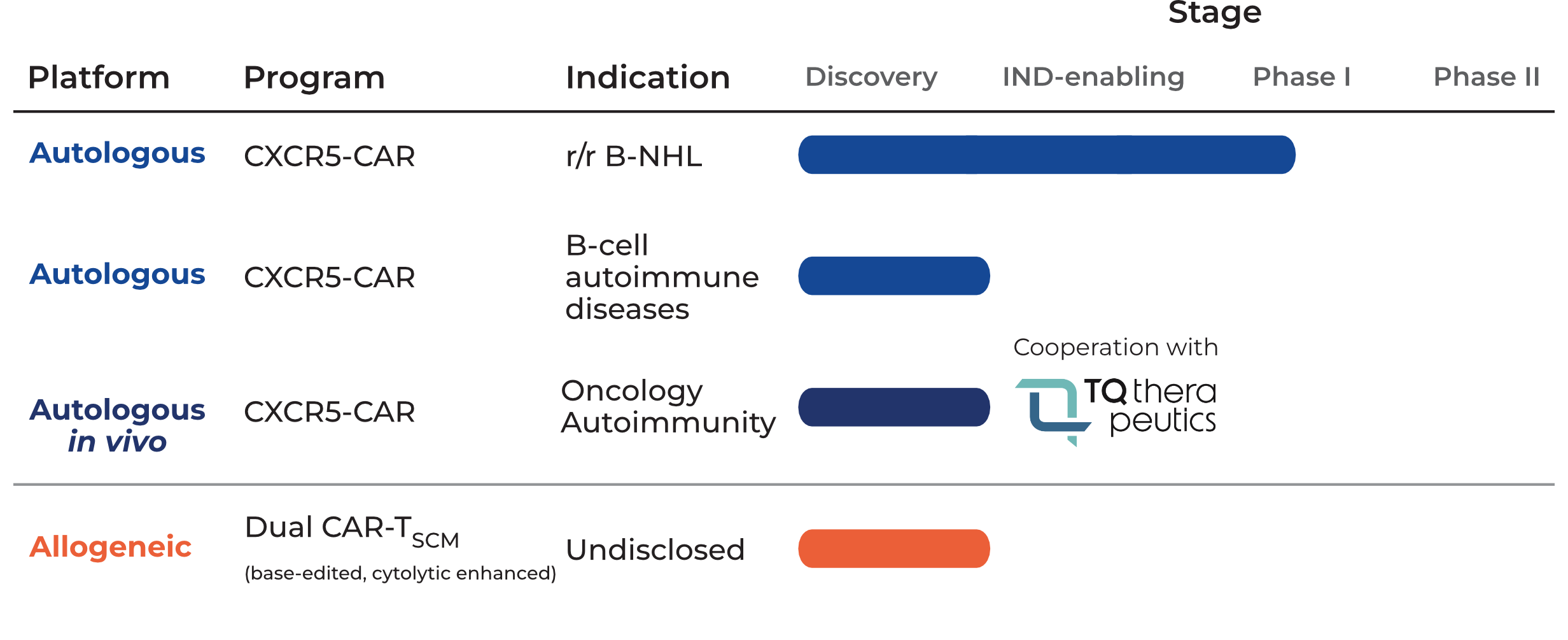
We are developing a cell therapy product candidate pipeline of first-in-class cellular immunotherapies for cancer and autoimmune diseases. Our proprietary portfolio is based on our ground breaking scientific achievements to address life-threatening diseases with unmet medical need and limited treatment options.
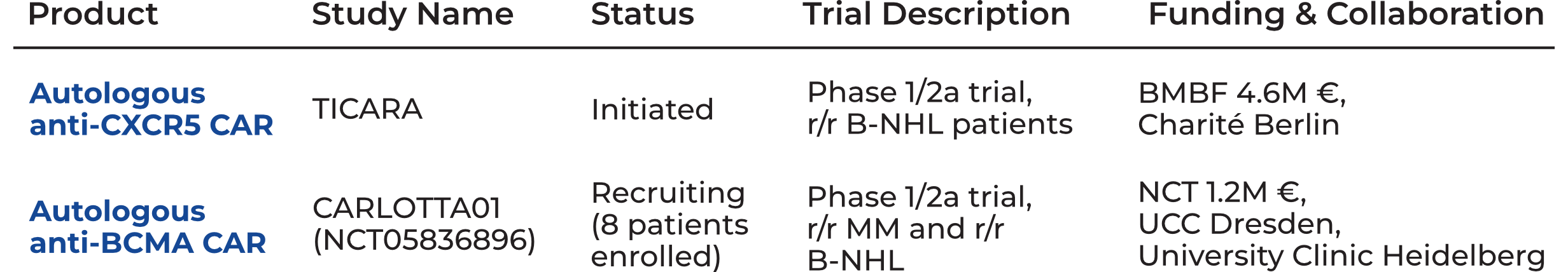
Clinical Trials
We developed a robust and reliable GMP-compliant process to enable the manufacturing of a clinical scale product. For advancing the anti-CXCR5 CAR into phase I/IIa, 4.6M€ BMBF-funding was secured to conduct an investigator-initiated trial in collaboration with physicians from the Charité-University Medicine Berlin. Patient enrolment is envisioned for the second half of 2026.
More details regarding the clinical trials CARTemis and/or their scientific founders are involved in can be found at clinicaltrials.gov.